
Quality
The Swiss Army Knife of Manufacturing
June 22, 2020

Our previous Finishing Friday post, Finishing Friday: Conversion Coating, presented many aspects of the process known as conversion coating. It’s a broad term, used to describe a surface treatment that expedites what occurs naturally to practically all metals, that is, oxidation. In steel and iron, this is called rust, but it also happens to aluminum and even stainless steel, albeit with less visible and dramatic results.
We talked about some of the more common ways to avoid this ugliness through processes such as black oxide and nickel plating. We touched briefly on one very important and specific class of conversion coatings: anodizing, which we refer to as the Big Kahuna of all conversion coatings. Now we’ll take a closer look at the anodizing process and applications of the more common types of anodizing.

Chromate conversion or “chem-film” is another aluminum coating option (and may also be applied after anodizing). So are powdercoating, painting, chrome, and electroless nickel, but anodizing is undoubtedly the go-to finishing option. That’s because anodizing makes aluminum…well, better. As explained earlier, it accelerates the oxidation process but does so in a tightly controlled manner, significantly thickening aluminum’s hard and naturally occurring oxide layer while giving manufacturers the option to also dye their parts in a rainbow of colors (for Type II anodizing, at least).
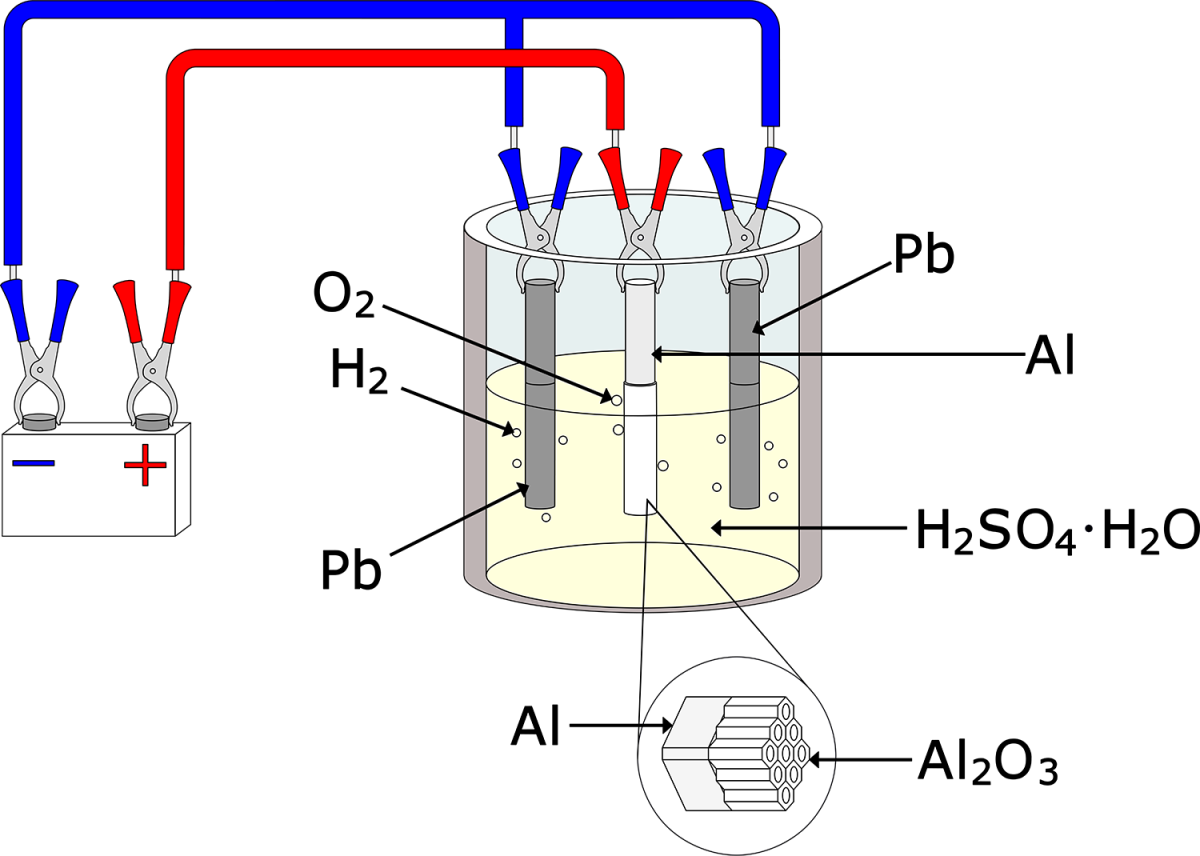
This process has been around a long time and some major variations have evolved. Anodizing’s first iteration—named the Bengough–Stuart process after the British defense engineers who invented it—was used to protect seagoing aircraft parts against corrosion. That was in 1923, and unfortunately for the inventors, their fame was short-lived; a competing process (and the one that’s most often used today) came along just four years later.

Sulfuric acid anodizing (the type that crushed Bengough and Stuart’s anodic dreams) is commonly referred to as Type II or decorative anodize. It’s up to ten times thicker than chromic acid anodize (around 0.0010″) and quite hard besides. As mentioned, Type II anodized parts are easily dyed to practically any color imaginable after leaving the tank (or left in their clear, un-colored state), after which they should be sealed to prevent bleeding. The carabiner hooks that many of us use as keyrings were Type II anodized, as are flashlight bodies, heatsinks, hot-rod parts, and hunting gear.

Thicker and harder yet is Type III, also known as…you guessed it, hardcoat. It’s two to three times the thickness of Type II, up to 60 Rc in hardness, and light gray to grayish-black in color. This is known as Class 1 hardcoat. Hardcoat can also be dyed anywhere from flamingo pink to a lustrous black (Class 2), although it will not boast the aesthetic qualities of its Type II counterpart. When left unsealed, Class 1 hardcoat exhibits increased abrasion resistance, whereas Class 2 hardcoat should be sealed using nickel acetate (preferred for outdoor use) or hot deionized water. Either class of hardcoat can be made to be fairly slippery when sealed with PTFE (Teflon). It is a favorite of the aerospace industry for its corrosion resistance and overall toughness, the medical industry for its sterilizability, and for all manner of mechanical engineering components. These include valve bodies, pistons, and other sliding components, as well as cams, gears, and hinged mechanisms.

There are a few more things to know about anodizing. First off, all types are electrically non-conductive. If your part requires a bare surface for a grounding screw, for example, that section will need to be masked off. However, there may be some difficulties in keeping the anodizing solution from seeping under the mask. Consistency and repeatability with masking can be challenging. At Prismier, we prefer to use to use an extra CNC machining operation to machine off the anodizing, if the part geometry allows for it. Additional labor expenses are incurred either way. Also, because half of all aluminum anodizing goes into the material, or “half in, half out” as some like to say, the buildup amounts given earlier are subjective—a flat metal stamping part with 0.0030″ of hardcoat on one side will only grow in thickness by 0.0015″. In contrast, a piston or other round part will grow the full 0.003″ (0.0015″ on each side). Hope that all makes sense, but give us a call if you have questions. We know anodizing!
If you'd like to know more, pick up the phone and call us at (630) 592-4515 or email us at sales@prismier.com. Or if you're ready for a quote, email quotes@prismier.com. We'll be happy to discuss your options.