
Quality
The Swiss Army Knife of Manufacturing
June 22, 2020
Tired of riding the stock market rollercoaster? Maybe you’re worried about inflation, or want some tangible, tradeable assets sitting in your safe for the coming zombie apocalypse. Perhaps gold is the solution. People have been using it as money for thousands of years, and until 1971, it served as the standard for monetary exchange throughout much of the world, the United States included.
Gold, platinum, and silver are the most well-known precious metals. According to Investopedia, they sit alongside palladium, rhodium, ruthenium, iridium, and osmium as the eight metals suitable for investment. Some of these are rare indeed—for every 19,700,000 tons of copper, only eight tons of iridium and one ton of osmium are produced yearly. And where gold currently sells for around $1750 per ounce, rhodium comes in at a whopping $14,250, making it the most precious of all precious metals.
What makes them so precious? It’s not their usefulness for bartering in a post-apocalyptic world (although it certainly wouldn’t hurt to have some gold coins stashed away were this unfortunate event to occur). No, it’s their metallurgical properties, which in nearly all cases make them valuable to manufacturers. Platinum, for example, is widely used in the medical industry for everything from pacemaker electrodes to cancer drugs. Catalytic converters would be far less efficient without palladium, while tossing a minuscule amount of ruthenium into the furnace increases titanium’s corrosion-resistance one-hundred-fold.

As noted, however, precious metals are darned expensive. This is why they are usually either A) manufactured into very tiny parts, as with the electrodes just mentioned, B) leveraged in small quantities as alloying elements, i.e., endowing titanium and countless other metals with desirable properties, or C) used to coat or plate stamped, formed, fabricated, and machined components. It’s this last application that we’re here to discuss for this edition of Finishing Friday.
If the extraterrestrials do intercept, they’ll find that those golden phonograph records are just as shiny as the day they left the plating factory. This brings us to one of the many reasons gold and other precious metals are so popular—their luster. If gold weren’t so beautiful and corrosion-resistant, it’s unlikely that humankind would have begun pounding it into jewelry and coinage millennia ago.
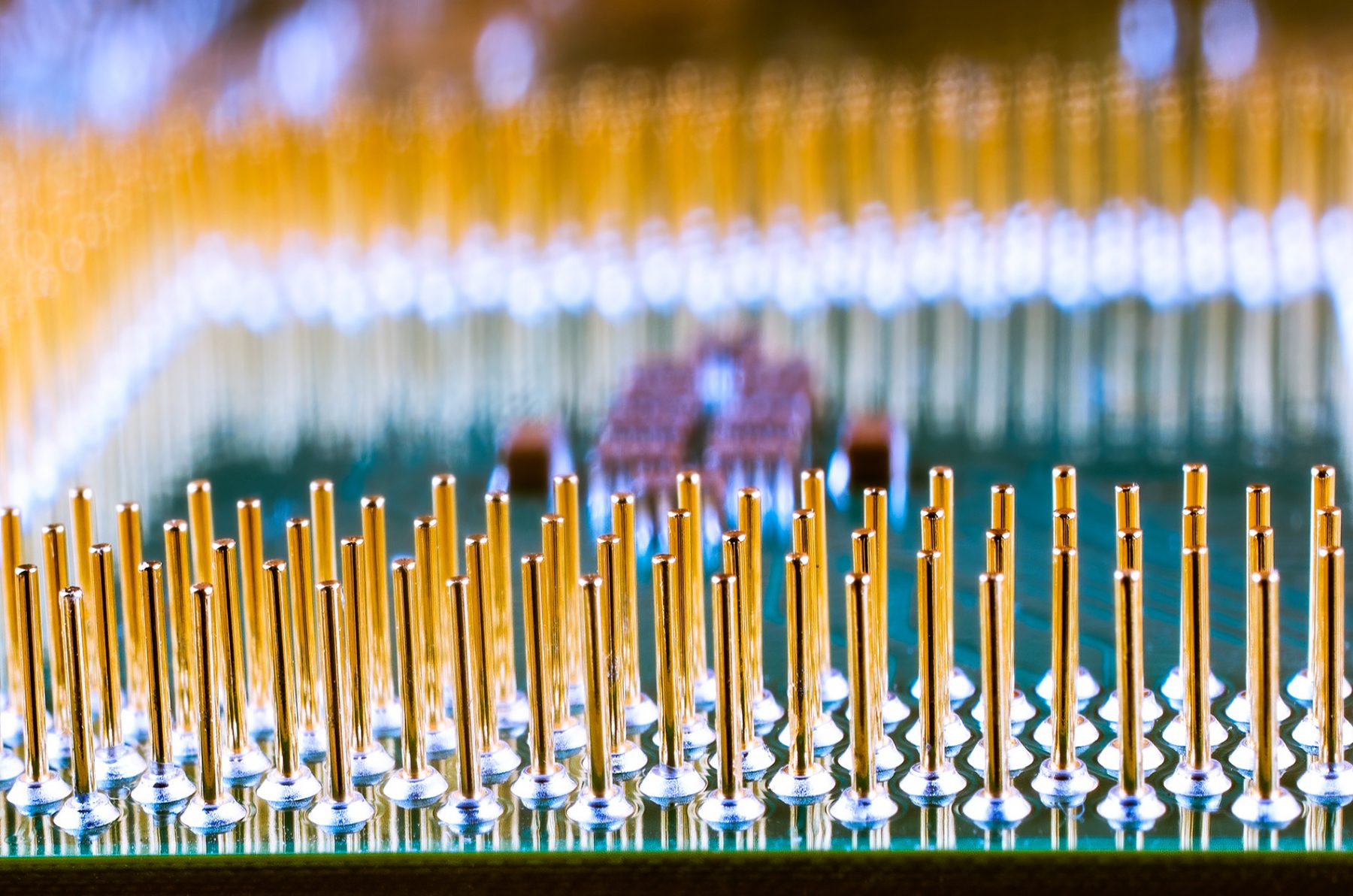
These two everyday uses still account for the majority of gold consumption today, but thanks to its excellent thermal and electrical conductivity, roughly one-third of all the world’s annual gold production is used to plate electronic connectors, components, and semiconductors. In fact, the average cell phone contains around $0.50 worth of gold, which is why it’s important to recycle your old cell phone when it’s time to upgrade. As with other precious metals, however, not much is needed to achieve the desired properties—according to military specification MIL-DTL-45204D, Class 00 calls for a whisper-thin 0.00002″ of gold plating while Class 6 deposits a minimum of 0.0015″, both much less than a human hair.
The various silver-plating processes fall under MIL-QQ-S-365D, not to mention ASTM B700 and AMS 2410. Depending on the specification, a potentially confounding variety of purities, lusters, and thicknesses are available, as well as different secondary treatments such as chromate and anti-tarnish. That brings us to silver plating’s Achilles Heel, an issue that anyone who’s spent hours cleaning Grandma’s silver flatware knows quite well—silver tends to discolor and darken, especially in humid environments or where sulfur-rich pollution (which is most of it) is a problem. Such cosmetic discoloration doesn’t reduce silver’s other benefits or mean that it’s unsuitable for long-term use, only that it grows less lustrous over time (a statement that also holds true for gold, assuming it’s less than 24-carat, i.e., completely pure). As the lucky one who did inherit Grandma’s silver flatware knows, you can remove the tarnish and restore the original lustrous form.

Hold on though—didn’t we state earlier that precious metals are non-reactive? That should make them impervious to such inconvenient ugliness, yes? But here’s the thing: because pure silver is so soft and malleable (like gold), Grandma’s prized forks and spoons are actually an alloy containing 7.5% copper, making them—you guessed it—sterling silver. And since the plating applied to all those gears and solar panels and gas turbine parts described a moment ago is often only 98 percent pure, some tarnishing is to be expected (hence the anti-tarnish offering).

It’s these first two—platinum followed by palladium—that receive the most attention from the manufacturing industry. Both have an affinity for hydrogen, so are common in automotive catalytic converters (as is rhodium), a factor that helps account for their high price tag. And since both are non-toxic and anti-bacterial, they are frequently used to plate objects that come into contact with the human body.
Palladium, for instance, often assumes gold’s traditional role in crowns, bridges, and other dental prostheses, while platinum is sometimes applied to titanium implants and used to make fine wires. Both are also radiopaque, making them easy to spot during x-ray imaging. And since neither metal tarnishes like sterling silver and is much harder besides, each is a favorite of the fine jewelry business.
Here are some highlights and typical uses for the other members of the platinum family:

With all these similarities, you might be wondering which one to choose? Great question. Of course, precious metal plating is a complex subject which requires knowledge of the product’s function and environmental conditions. In addition, the available budget, compatibility with the base metal, and other factors may influence the final outcome. The experts here at Prismier can help you navigate the options and tradeoffs.
As with all plating processes, the parts must be thoroughly cleaned and possibly polished before placement into the tank, barrel, or rack. They could also require pre-plating with a nickel “strike” layer to increase adhesion, are frequently plated afterward with other precious metals, and may need post-plating heat treatment to avoid hydrogen embrittlement. Again, it’s a deep topic. If you’d like to find out more about precious metals or just want to chat about the possibility of gold-plated record albums being found in distant galaxies, you know where to find us.
If you'd like to know more, pick up the phone and call us at (630) 592-4515 or email us at sales@prismier.com. Or if you're ready for a quote, email quotes@prismier.com. We'll be happy to discuss your options.